Universidad de Valencia
Facultad de
Odontología
Diseño y Presentación de Trabajos
Científicos
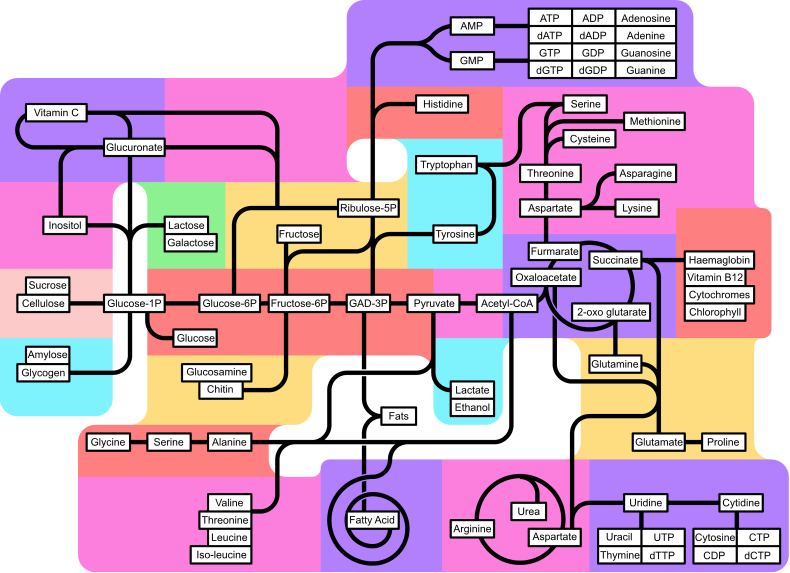
METABOLISM
Ramteen Ahrabi
Valencia,
12/04/2009
DEDICATORIA
Este
trabajo se lo quiero dedicar a todas las personas en el área académica
que han hecho posible que aprendiera toda la información necesaria para
entrar en el curso de Odontología en la Universidad de Valencia: los
profesores del colegio Entrenaranjos, el director, y mis profesores
particulares que me han guiado mucho en este camino.
También me
gustaría incluir en esta dedicatoria a todos los científicos en este
mundo que han hecho todos estos descubrimientos sobre el cuerpo humano,
incluso sobre el Metabolismo, que ha ayudado mucho a prolongar la vida
de muchísimas personas.
ÍNDICE
1. Introduction
..a) History of the Term
2.
Metabolism
..a) Primary
Biochemicals
..b) Energy
Transformations
..c)
Catabolism
..d) Anabolism
3.
Conclusions
4. Resources
5. Glossary
6. Special
Thanks
*Words marked with an asterisk appear
in the glossary
INTRODUCTION
Metabolism is a series of processes
which take place within all living organisms. These processes are
chemical reactions, and their primary objective is maintaining life via
growth, reproduction, structure maintenance, and adequate environment
response. The chemical reactions which constitute Metabolism are divided
into two specific categories: Catabolism, which consists of the breaking
of organic matter for various procedures; and Anabolism, characterised
by the construction of cell components such as proteins and nucleic
acids.
Each chemical reaction concludes with the transformation
of one chemical into another by a sequence of Enzymes. The majority of
enzymes are proteins, and they serve to couple unfavourable reactions
with desirable ones, since they act as catalysts to speed up the
procedure of the reactions. This increase in speed is crucial to
metabolism, as there are some certain reactions that would just slow
down to the point of ceasing entirely in the absence of a suitable
enzyme, which can sometimes prove to be dangerous for an organism’s
life.
Metabolism also serves to differentiate nutritious
substances from poisonous ones, and the speed of metabolism, known as
the Metabolic Rate, determines how much food an organism requires in
order to survive.
Different organisms do not necessarily play a
role in the difference between chemical reactions. For instance, the
specific set of Carboxylic Acids* which act as intermediates in the
Citric Acid Cycle* (otherwise known as the Krebs Cycle) are present in
all organisms, from the unicellular bacteria Escherichia Coli to huge
organisms like elephants (Smith, Morowitz, 2004, 13168-73).
HISTORY OF THE TERM
The word “Metabolism” is derived from the Greek
“Metabolismos”, which means “change” or “overthrow”, and the history of
the scientific study of metabolism itself spans several centuries and
has gone from studying whole animals in early studies to examining
individual metabolic reactions in modern biochemistry. Ibn al-Nafis
stated that “the body and it’s parts are in constant nourishment, so
they are inevitably undergoing a permanent change” (Dr. Abu Shadi
Al-Roubi, 1982). The very first controlled experiments in
human metabolism were published by Santorio Santorio, who described that
he weighed himself before and after eating, sleeping, sex, working,
fasting, drinking and excreting (Eknoyan, 1999,
226-33).
METABOLISM
PRIMARY BIOCHEMICALS
The
structures of animals, plants and microbes, in their majority, are made
up of three basic classes of molecule, namely Amino Acids, Carbohydrates
and Lipids/Fats. Metabolism mainly focuses on either creating these
substances for the construction of cells and tissues, or breaking them
down for the digestion of food and use as a source of energy. Certain
biochemicals can be combined to make different polymers, such as DNA, or
proteins, which are extremely essential for living
organisms.
Amino Acids combine to create proteins in a linear
chain joined together by peptide bonds. Proteins are essential for
organisms to the point that many of them are enzymes which catalyse
chemical reactions. Other functions of proteins include cell signalling,
immune responses, cell adhesion, active transport across or through
membranes, and the cell cycle (Nelson, Cox, 2005, 841).
Lipids
are the most diverse group
of biochemicals, and they are usually used as part of biological
membranes or as a source of energy. Lipids are
normally differentiated
between hydrophobic or amphipathic biological molecules that will
dissolve in organic solvents. The fats that lipids compose are a large
group of compounds that contain fatty acids and glycerol, and are named
according to how many fatty acids are attached to the glycerol molecule:
for instance, if there are three fatty acids, they form a
Triacylglyceride.
Carbohydrates are straight-chain ketones or
aldehydes with many hydroxyl groups in straight chains or in rings. They
are the most abundant biochemicals, and their primary functions are
storage and transportation of energy, and structural composition. The
basic units are called monosaccharides, which can be linked to form
polysaccharides in any way possible (Raman et al., 2005,
817-24).
Nucleotides, chained
together, from the polymers DNA* and RNA*. They are critical for the use
and storage of genetic information through the processes of
transcription and protein biosynthesis.
ENERGY TRANSFORMATIONS
One
notable example of energy transformation is Oxidative Phosphorylation,
in which electrons obtained from food molecules are transferred to
oxygen and the energy released is used to make ATP. In Eukaryotes, this
is done by a series of proteins in the membranes of mitochondria called
the electron transport chain. In Prokaryotes, those proteins are found
in the cell’s inner membrane (Hosler et al., 2006, 165-87). These
proteins then use the energy
released from passing electrons from reduced molecules like NADH onto
oxygen to pump protons across a membrane (Schultz, Chan, 2001, 23-65).
This creates a proton
concentration difference across the membrane and generates an
electrochemical gradient, which drives the protons back into the
mitochondrion through the base of an enzyme called ATP Synthase. The
flow of protons makes the stalk subunit rotate, which causes the active
site of the synthase domain to change shape and phosphorylate Adenosine
Disphosphate, subsequently turning it into ATP (Dimroth et al., 2006,
276-82).
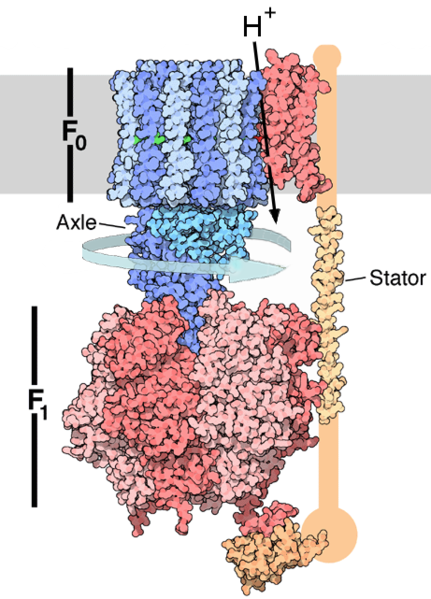
Figure 1. Structure of
ATP Synthase. (Goodsell, 2005)
Another form of
energy transformation is Chemolithotrophy, which is found in Prokaryotes
and in which energy is obtained from the oxidation of inorganic
compounds. These organisms can use hydrogen, sulphur compounds, ferrous
iron or ammonia as sources of reducing power and they gain energy from
the oxidation of these compounds with electron acceptors like oxygen or
nitrite. These processes have proven to be very important for many
biogeochemical cycles (Conrad, 1996, 609-40).
CATABOLISM
Catabolism is the branch of Metabolism which
consists in the breaking of macromolecules into smaller structures and
the usage of these smaller substances for various processes, such as
providing energy and necessary components for Anabolism. From organism
to organism, the processes of Catabolism vary drastically. In animals,
Catabolism is divided into three stages: in the first, the large organic
materials, like proteins and lipids, are digested into their smaller
respective components outside cells; in the second, these smaller
components are taken by cells and broken into even smaller structures,
usually Acetyl Coenzyme A* (CoA); and in the third, the Acetyl group from
CoA is oxidised into water and carbon dioxide in the Krebs Cycle and
electron transport chain, releasing the stored energy by reducing the
coenzyme NAD+ into NADH.
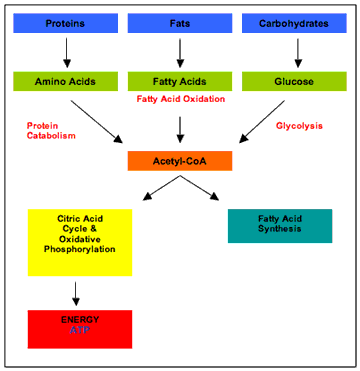
Figure 2. Schematic diagram of
Catabolism (Hamilton, 2008)
ANABOLISM
Anabolism, however, is the branch of Metabolism
which consists of the usage of energy liberated by Catabolic processes
to synthesise more complex structures. It is divided into three stages:
first, the production of the precursors, such as amino acids and
nucleotides; second, their activation into reactive forms using energy
from ATP; and third, their assembly into complex molecules such as
proteins and nucleic acids.
Like in Catabolism, this process
differs in different organisms. Autotroph organisms, for instance, can
build the complex molecules in cells like proteins from simple molecules
like carbon dioxide. Heterotroph organisms, on the other hand, cannot.
Rather, they need a source of more complex substances like amino acids
in order to make these complex molecules. Another way to classify
organisms is their source of energy: while Photoautotrophs and
Photoheterotrophs obtain energy from light (hence the prefix, “Photo”),
Chemoautotrophs and Chemoheterotrophs obtain their energy from inorganic
oxidation reactions (the prefix, “Chemo”, derived from chemical
reaction).
CONCLUSIONS
Studies of the human metabolism have proven
many times over that it is the combination of all the processes
necessary for the survival of the human race. Several illnesses such as
Hepatitis* can cause a problem in metabolism, and thus possibly lead to
the death of the patient.
But even though this is a fact, not
many people in this world take much notice of the warnings given by
doctors against the consumption of specific foods which may lead to the
damaging of metabolism. One notable example is the excessive consumption
of fatty foods, such as chocolate, butter, and mainly dairy products,
but basically most foods which contain high amounts of sugar, and due to
the severe addiction of some people to these foods, it is not as simple
as it seems to simply ban their commercialisation.
On the other
hand, activists have also worked hard to persuade the public to look
after their bodies more efficiently, such as publishing magazines and
books dedicated to this subject. These magazines serve as guides for a
good diet for most people, and even suggest ways of increasing someone’s
metabolism, such as a sufficient amount of exercise and physical
activity in order to burn calories and prevent the creation of an excess
of fat which may lead to the slowing of the blood stream and an eventual
heart attack, which might or might not be
fatal.
RESOURCES
- Conrad R. 1996. Soil microorganisms as
controllers of atmospheric trace gases: New York: Microbiol Rev.
609-640.
- Dimroth P. et
al. 2006. Catalyctic and mechanical cycles in F-ATP Synthases. Fourth in
the Cycles Review Series: EMBO Rep, 276-282.
- Dr. Abu Shadi
Al-Roubi. 1982. Sympiosum on Ibn al Nafis: Kuwait.
- Eknoyan G. 1999.
Santorio Sanctorius (1561-1636) - founding father of metabolic balance
studies: Am J Nephrol, 226-233.
- Goodsell D. S. 2005. Molecule
of the
Month: New Jersey: Protein Data Bank.
- Hamilton M. 2008. Obesity
and Diabetes: A Canadian
Epidemic: British Colombia: The Science Creative Quarterly.
-
Hosler J. et al. 2006. Energy transduction: proton
transfer through the respiratory complexes: Annu Rev Biochem,
165-187.
- Nelson D.,
Cox M. 2005. Lehninger Principles of Biochemistry: New York: W. H.
Freeman and Company, 841.
- Raman
R. et al. 2005. Glycomics: an integrated systems approach to
structure-function relationships of glycans: Nat Methods,
817-824.
- Schultz
B., Chan S. 2001. Structures and proton-pumping strategies of
mitochondrial respiratory enzyme: Annu Rev Biophys Biomol Struct,
23-65.
- Smith E., Morowitz H.
2004. Universality in Intermediary Metabolism: Chicago: Proc Natl Acad Sci,
13168-13173.
GLOSSARY
Acetyl Coenzyme A. A molecule used to
convey carbon atoms within the acetyl group to the Citric Acid Cycle to
be oxidised for energy production.
Carboxylic Acid.
Organic acids characterised by the presence of a carboxyl
group.
Citric Acid Cycle. A series of enzyme-catalysed
chemical reactions of central importance in living cells that use oxygen
as part of cellular respiration.
DNA. Deoxyribonucleic
acid, a nucleic acid that contains the genetic instructions used in the
development and functioning of all known living organisms and some
viruses.
Hepatitis. An injury to the liver characterised
by the presence of inflammatory cells in the tissue of the
organ.
RNA. A molecule consisting of a long chain of
nucleotide units, each made of a nitrogenous base, a phosphate and a
ribose sugar.
SPECIAL THANKS
I want to give my special thanks to those
from school who first taught me the basics about writing a special
document, and to those from the University who amplified my knowledge
enough and gave me so many hours of class in order for me to produce
this work, even while they were out of the University.


